文献:Ligand-Modified Erythrocyte Membrane-Cloaked Metal−Organic Framework Nanoparticles for Targeted Antitumor Therapy
文献链接:https://pubmed.ncbi.nlm.nih.gov/32804508/
作者:Yixuan Lin,Yuping Zhong, Yongda Chen, Lin Li, Guoping Chen, Jiaxian Zhang, Pei Li,Chunhua Zhou, Yangwen Sun, Yan Ma, Zhiyong Xie, and Qiongfeng Liao
相关产品:DSPE-PGE2000-cRGD 磷脂-聚乙二醇2000-cRGD肽
原文摘要:
Systemic chemotherapy for treating tumors often leads to serious systemic side effects and affects patient compliance. Although the emerging technology of drug delivery systems (DDSs) can deliver the required cargo to tumor sites, DDSs are limited due to insufficient targeting ability or deficient pharmacokinetics. Herein, we assembled a novel targeting DDS for precision tumor therapy by applying a tumor-targeting polypeptide cyclic RGD (cRGD)-modified erythrocyte membrane (eM-cRGD) cloaked on zeolitic imidazolate framework-8 (ZIF-8) nanoparticles (NPs) with encapsulated doxorubicin (DOX).For a mass ratio of ZIF-8:DOX = 1:1, the loading capacity was up to 49%. The nanoscale-sized targeting DDS promoted NP accumulation in tumor tissues via enhanced permeability and retention (EPR) effects, and the NPs actively targeted ligands and were then transferred to endosomes. The pH-sensitive carriers released higher DOX levels under the low pH mimicking that of a tumor microenvironment and tumor intracellular organelles, allowing enhanced inhibition of cancer cell growth. The cumulative release rate of DOX from DOX@ZIF-8 NPs reached 82.8% at 48 h in acidic conditions of pH = 5.0, while the cumulative release rate of DOX from the DOX@ZIF-8 NPs reached only 24.92% at pH = 7.4. The internalization of the DDS was approximately 44.35% that of the unmodified DDS by immune cells, as confirmed by flow cytometry. In vivo studies verified that the RGD-modified DDS had the ability to prolong blood circulation (t1/2 = 6.81 h), enhancing the tumor-specific accumulation of
NPs by means of the integrin αvβ3 receptor-mediated pathway, which was further valuated in mice bearing human cervical cancer(HeLa) cells, and yielding a significant antitumor effect; the tumor inhibition rate was as high as 85.46%. Under the same conditions,the blood circulation half-life of the unmodified DDS was only 3.22 h, and the tumor inhibition rate of free DOX was 81.34%.Moreover, the RGD modified with a carrier could achieve a satisfactory chemotherapeutic effect while minimizing side effects. Insummary, our novel targeting DDS could contribute to the development of intelligent DDSs for tumor precision therapy.
DSPE-PGE2000-cRGD:DSPE是一种磷脂成分。它包含两条长的疏水脂肪酸链(硬脂酸),这使得 DSPE 具有很强的疏水性。PGE是聚乙二醇,分子量为 2000。PEG 是亲水性聚合物,能够改善材料的亲水性,并且可以减少蛋白质吸附,增强在生物环境中的稳定性。它连接 DSPE 和 cRGD,使得整个分子兼具亲水性和疏水性,有利于在水性环境中形成稳定的纳米结构,如胶束或脂质体。cRGD(环状精氨酸-甘氨酸-天冬氨酸)是一种具有靶向功能的短肽。RGD 序列是细胞外基质和细胞表面整合素受体识别的重要位点。cRGD 肽通过其特殊的环状结构增强了对整合素受体的亲和力和选择性。基于DSPE-PGE2000-cRGD的特点,该文献合成DOX@ ZIF-8@eM-cRGD NPs路线如下:
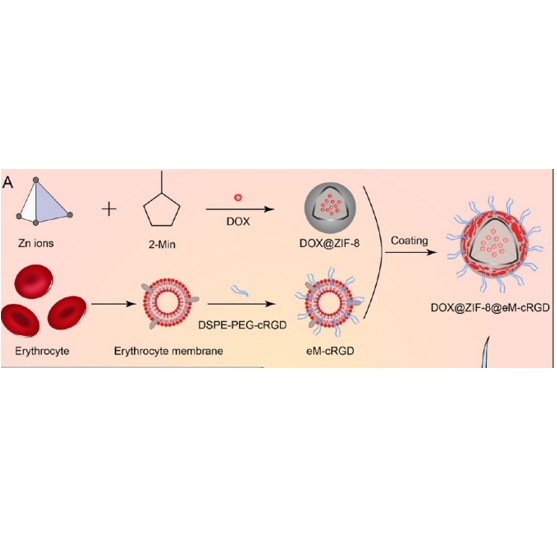
图:合成流程
RGD肽对NPs进行表面修饰:
将PBS制备的DSPE-PEG2000-cRGD溶液加入制备的红细胞鬼液中,孵育,离心,去除游离的DSPE-PEG2000-cRGD,获得cRGD修饰的eMs(eM-cRGD)。然后,挤压细胞膜。将ZIF-8或DOX@ZIF-8 NP溶液进行涡旋,eM-cRGD与已知的NP-膜质量比缓慢混合并持续混合超声。悬浮液经超声处理后,使用迷你挤压机连续挤压多次,该文献制出eM-cRGD包裹DOX@ZIF-8 NPs。DOX@ZIF-8@eM-cRGD NPs均匀分散在PBS中以供后续使用。

图:电镜图像
结论:
该文献成功制备了基于DSPE-PGE2000-cRGD合成DOX@ ZIF-8@eM-cRGD NPs,这是一种建立了MOF的酸响应纳米DDS。DOX@ ZIF-8@eM-cRGD NPs可以在注射后将化合物转移到tumour部位,增加化合物在tumour部位的积累,并进一步增加tumour生长抑制。综上,这种新型靶向DDS可能有助于开发用于tumour的智能DDSs。

 2025-02-10 作者:ws 来源:
2025-02-10 作者:ws 来源:

