原文献:聚乙二醇衍生物的合成和特性研究
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=43db981359b6e853194c58c41d57df37&site=xueshu_se&hitarticle=1
作者:周维
原文摘要:More and more protein and peptide drugs are being used for the treatment of human diseases. Compared with other synthetic chemical drugs, they have the disadvantages of easily causing immune reactions in the body, short half-life in the body, and easy hydrolysis and denaturation in the body. Chemical modification, as an emerging technology, can improve the above-mentioned adverse characteristics. This article mainly optimized the synthesis of a PEG modifier - mPEG NHS. The modification conditions were optimized using bovine serum albumin (BSA) and lysozyme as model proteins, and the modified proteins were separated by chromatography. The synthesis of mPEG NHS is mainly obtained through two reactions. The first step is the esterification reaction between mPEG and succinic anhydride to obtain mPEG SA. The second step is the reaction between mPEG SA and NHS (N-hydroxythiosuccinimide), catalyzed by the dehydrating agent DCCI (N.N '- cyclohexyl carbodiimide) to obtain mPEG NHS. By optimizing the reaction conditions, the conversion rate of mPEG and the purity of mPEG NHS were both improved. The optimized reaction conditions are as follows: (1) Pyridine is used as a catalyst for esterification reaction, with an acid alcohol ratio of 10:1 and a reaction time of 3 hours; (2) Dehydration reaction time is 25 hours, temperature is 40 ℃, and the molar ratio of reactants mPEG SA: NHS is 1:2.5. The conversion rates of the optimized two-step reaction were 60.1% and 56.0%, respectively. MPEG NHS modified proteins yield proteins with different modification rates under different reaction conditions, and optimized reaction conditions can result in modified products with higher amino modification rates. The optimal modification reaction conditions are: reaction time of 10 minutes, protein to modifier mass ratio of 1:5, using borax buffer at pH 9.0. Under optimized conditions, a product with a modification rate of 47.5% can be obtained. Due to the presence of proteins with and without modifiers in the protein solution obtained from the modification reaction, they can be separated by chromatography. The lysozyme modified product was subjected to sephadex G-75 gel chromatography and Deae sepharose CL-6B cation exchange chromatography; The BSA modified product was subjected to a combination of Sephadex G-100 and Q-Sepharose anion exchange chromatography. Using SDS-PAGE electrophoresis to detect the separated products, it was demonstrated that unmodified proteins were separated from modified proteins.
蛋白质多肽类药物与其它合成化学药物相比,易引起机体的免疫反应,体内半衰期短,在体内易水解、变性等缺点。化学修饰改善上述不良特性。研究优化合成了一种PEG衍生物——mPEG-NHS,采用牛血清白蛋白BSA和溶菌酶作为模式蛋白对其修饰条件进行了优化,并用层析法分离修饰后蛋白质。制备方式如下:
mPEG-NHS的合成主要通过两个反应得到,第一步是mPEG同丁二酸酐之间的酯化反应,得到mPEG-SA,第二步是mPEG-SA同NHS(N-羟基硫代琥珀酰亚胺)反应,在脱水剂的催化下得到mPEG-NHS。通过优化反应条件使得mPEG的转化率和mPEG-NHS的纯度都得到提高。
优化后反应条件分别为:(1)酯化反应采用吡啶为催化剂;(2)脱水反应时间25h,温度40℃,优化后的两步反应的转化率分别为60.1%和56.0%。由于修饰反应得到的蛋白质溶液中含有连接有修饰剂的蛋白质和未连接修饰剂的蛋白质,可通过层析的方法将它们分离开。
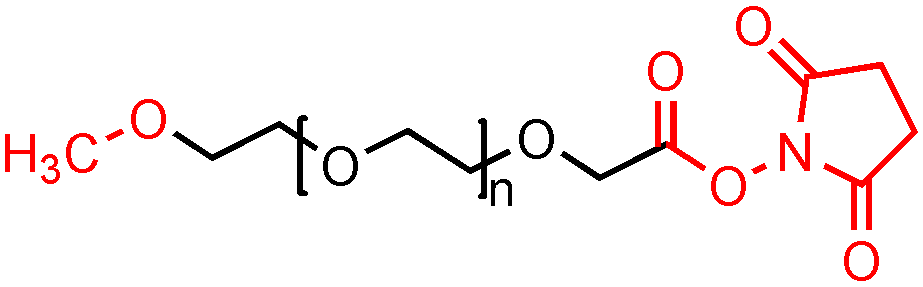
图:mPEG-NHS结构式
结论: mPEG-NHS修饰蛋白质在不同的反应条件下得到不同修饰率的蛋白质,优化反应条件后能得到更高氨基修饰率的修饰产物。用SDS-PAGE电泳检测分离产物,证明未修饰的蛋白质同被修饰的蛋白质被分离开来。

 2024-12-18 作者:ZJ 来源:
2024-12-18 作者:ZJ 来源:

