文献:Antisense oligonucleotides-Laden UiO-66@Au nanohybrid for enhanced radiotherapy against hypoxic tumor by dual-inhibition of carbonic anhydrase IX
文献链接:https://www.sciencedirect.com/science/article/abs/pii/S2352940721002651
作者:Kai Wang , Shuaishuai Ding , Lijuan Zeng, Jingrong Zhou , Yuhua Cao , Jiaqian Wu , Lu Lu , Xiu-wu Bian , Gan Tian
相关产品:Thiolated polyethylene glycol (PEG-SH, Mw: ~5000 g mol−1)(聚乙二醇-巯基)
原文摘要:
Hypoxia-acquired radioresitance is considered to be a major factor responsible for the clinical failure of radiotherapy. Carbonic anhydrase IX (CA IX), a hypoxia-induced cell-surface enzyme involved in pH regulation of hypoxic solid tumors, has been acknowledged as a desirable target for cancer therapy. Herein, we developed a dual exogenous/endogenous CA IX inhibition strategy using core/satellite-like metal-organic framework (UiO-66)/gold nanoparticles (Au NPs) nanohybrids as a therapeutic platform for hypoxia-relief enhanced RT of triple negative breast cancer. The UiO-66 matrix supported the Au NPs generation in situ and would decompose inside tumor cells by the high-concentration phosphates to release p-phthalic acid (PTA), a original building skeleton of the UiO-66 matrix, to inhibit CA IX, while the decorated Au NPs served as radiosensitizers to potentiate the sensitivity of tumor cells to X-ray and yet provided active sites for CA IX antisense oligonucleotide (ASO) loading via formation of stable Au-S bond to knockdown CA IX. The dual inhibitory manner, exogenous inhibition from PTA and endogenous inhibition from CA IX ASO, largely alleviated the hypoxia-induced resistance and synergized with Au NPs-mediated radiosensitization to afford super therapeutic outcome in vitro and in vivo, supporting the feasibility of our synergistic RT strategy in hypoxic tumor management.
PEG-SH:PEG:是聚乙二醇(Polyethylene Glycol),它是一种由环氧乙烷与水或乙二醇逐步加成聚合得到的聚合物。其化学结构是由重复的乙二醇单元(-CH₂CH₂O -)组成,分子链的两端可以根据聚合反应的条件和后续处理,呈现不同的化学基团。SH:代表巯基(-SH),是一种含有硫和氢的官能团。巯基具有较强的反应活性,能够参与多种化学反应,如氧化还原反应、与金属离子配位反应和与含有活性双键的化合物进行加成反应等。PEG - SH 是聚乙二醇分子的一端或两端连接了巯基的化合物。通常是通过在 PEG 分子的末端引入含有巯基的小分子化合物来实现这种修饰。基于PEG-SH的性能,UiO-66/Au-ASO/PEG(UAAP)合成如下:
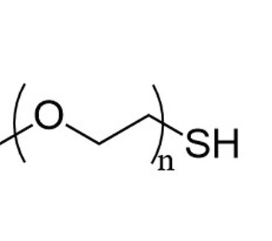
图:PEG-SH的结构式
UiO-66/Au-ASO/PEG(UAAP)合成如下:
合成 UiO-66:称取对苯二甲酸溶解于DMF 中,加入圆底烧瓶中,搅拌;称取八水氯氧化锆溶解于DMF 中,将其加入到上述圆底烧瓶中,继续搅拌 5min,使二者充分混合均匀,再加入乙酸。将圆底烧瓶置于油浴中,搅拌反应。反应结束后,将产物离心,分别使用 DMF、去离子水洗涤,然后在真空干燥箱中干燥,得到 UiO-66 纳米颗粒。
制备 UiO-66/Au:将 UiO-66 分散于去离子水中,充分搅拌后,滴加氯金酸溶液,常温搅拌。加入冰冷的硼氢化钠水溶液,继续搅拌反应。反应完成后,离心收集固体,用去离子水洗涤,最后重新分散于去离子水中备用,得到 UiO-66/Au。
偶联 ASO:将 UiO-66/Au 水溶液与 ASO 水溶液混合,反应,使 ASO 通过 Au-S 键与 UiO-66/Au 表面的金纳米粒子偶联,得到 UiO-66/Au-ASO。
PEG 化修饰:向 UiO-66/Au-ASO 溶液中加入 PEG-SH,反应。反应结束后,通过离心等方法进行固液分离,得到 UiO-66/Au-ASO/PEG 纳米杂化物 。

图:机制示意
结论:
该文献成功制备出基于PEG-SH合成的UiO-66/Au-ASO/PEG(UAAP)。该纳米复合材料具有小分子抑制剂和ASO对CA IX的外源性/内源性双重靶向抑制作用,以及高纳米材料赋予的低氧增强RT的放射敏感性。UAAP一旦进入tumour细胞,UiO-66基质就会被细胞内高浓度磷酸盐分解,先前整合的功能片段会发挥各自的功能,相互协同,提供Therapeutic effect 。

 2025-04-10 作者:ws 来源:
2025-04-10 作者:ws 来源:

