文献:R848 Adjuvant Laden With Self-Assembled Nanoparticle-Based mRNA Vaccine Elicits Protective Immunity Against H5N1 in Mice
文献链接:https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.836274/full
作者:Xinyu Zhuang,Luer Chen2,Songhui Yang,Shengnan Xia,Zhiqiang Xu,Tong Zhang,Boyu Zeng,Tong Yu,Ning Yu,Wei Wang,Huijun Lu,Mingyao Tian,Ningyi Jin
相关产品:DSPE-PEG-Mannose 磷脂-聚乙二醇-甘露糖
原文摘要:In order to perfect the design strategy of messenger RNA (mRNA) vaccines against the H5N1 influenza virus, we investigated whether different antigen designs and the use of adjuvants could improve the immune effect of mRNA vaccines. We designed three different forms of antigen genes, including Flu [H1/H3/H5/B-HA2(aa90~105)-M2e(24aa)], Flu-Fe (Fe, ferritin), and CD5-Flu-Fe (CD5, a secretion signal peptide). Meanwhile, R848 (Requimod) was selected as the adjuvant of the mRNA vaccine. We prepared cationic lipid nanoparticles for mRNA delivery, named LNP-Man (mannose-modified lipid nanoparticles). Cell transfection results showed that Flu-Fe/CD5-Flu-Fe containing ferritin could express the target antigens HA2 and M2e more efficiently than Flu. In the mice immune experiment, five immune groups (LNP-Man/Flu, LNP-Man/Flu-Fe, LNP-Man/CD5-Flu-Fe, LNP-Man/Flu-Fe+R848, and LNP-Man/CD5-Flu-Fe+R848) and two control groups (LNP-Man, PBS) were set up. After being infected with the 1×LD50 H5N1 avian influenza virus, the survival rate of the mice in the LNP-Man/CD5-Flu-Fe, LNP-Man/Flu-Fe+R848, and LNP-Man/CD5-Flu-Fe+R848 were 100%. More importantly, in LNP-Man/Flu-Fe+R848 and LNP-Man/CD5-Flu-Fe+R848 groups, there was no residual virus detected in the mice lung tissue on the 5th day postchallenge. Overall, this study provides a new idea for the design of H5N1 avian influenza virus mRNA vaccines in terms of antigen designs and adjuvant selection.
DSPE-PEG-Mannose是一种功能性生物分子材料,其全称为二硬脂酰磷脂酰乙醇胺-聚乙二醇-甘露糖。它由三部分组成。DSPE部分是一种常用的磷脂,具有良好的生物相容性和膜融合能力,能使该分子更好地与生物膜相互作用;PEG链则提供了亲水性和柔性,可有效降低分子的免疫原性,延长其在体内的循环时间;甘露糖是一种具有特异性识别功能的糖类,能与细胞表面的甘露糖受体结合。它可以用来制备脂质体,将化合物包裹其中,通过甘露糖与特定细胞的靶向结合,实现化合物的准确递送。该文献制备了用于mRNA递送的阳离子脂质纳米颗粒,命名为LNP-Man(甘露糖修饰脂质纳米颗粒)。制备过程如下:
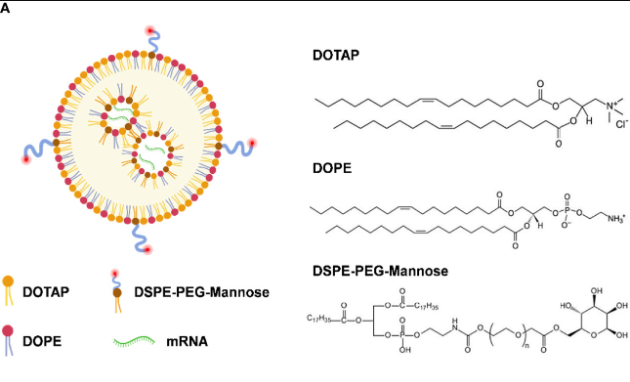
图:阳离子脂质纳米颗粒的合成示意图
LNP-Man /信使rna制备
mRNA被包裹在LNP-Man(甘露糖修饰脂质纳米颗粒,DOTAP: DOPE: dspe-peg -甘露糖(mol/mol)=50:50:1)中,通过微流控装置将mRNA水溶液与溶解在乙醇中的脂质溶液快速混合,进行自组装过程。用注射泵以比例灌注乙醇和水相。配方使用10 kD超离心过滤器(Millipore, Billerica, MA, usa)进行浓缩。按指定的N:P摩尔比制备LNP-Man/mRNA (N,氮在DOTAP上;P, mRNA上的磷酸盐)。假设每个核苷酸的平均摩尔质量为330 Da,用于计算DOTAP和RNA之间的摩尔比,然后根据Malvern Zetasizer Nano ZS90 (Malvern Panalytical, Westborough, MA, usa)测量颗粒大小和zeta电位。最后用透射电镜(TEM)和扫描电镜(SEM)观察其形态。

图:LNP-Man和mEGFP按N/P摩尔比1:1、2:1、3:1、4:1、5:1和6:1混合。转染A549细胞24 h后,荧光显微镜观察结果。
结论:DSPE-PEG-Mannose参与制备的阳离子脂质纳米颗粒(LNPs)在mRNA递送领域成为载体系统。其大小通常处于几十到几百纳米的范围,这一适中的粒径有助于避免被快速清除出循环系统,延长在体内的循环时间,还能有效抵达靶细胞。表面带正电荷是LNPs的物理特性,这种正电性使其能够与带负电的mRNA通过静电相互作用紧密结合,形成稳定的复合物,从而保护mRNA免受核酸酶的降解。而且,带正电的表面有利于与带负电的细胞膜相互作用,促进颗粒被细胞内吞,提高mRNA的递送效率。

 2025-04-09 作者:ZJ 来源:
2025-04-09 作者:ZJ 来源:

