文献:Precision design of nanomedicines to restore gemcitabine chemosensitivity for personalized pancreatic ductal adenocarcinoma treatment
文献链接:https://www.sciencedirect.com/science/article/abs/pii/S014296121730813X
作者:Xiao Zhao , Xiuchao Wang , Wei Sun , Keman Cheng , Hao Qin ,Xuexiang Han, Yu Lin , Yongwei Wang, Jiayan Lang , Ruifang Zhao, Xiaowei Zheng,Ying Zhao, Jian shi, Jihui Hao, Qing Robert Miao , Guangjun Nie, He Ren
相关产品:
原文摘要:Low chemosensitivity considerably restricts the therapeutic efficacy of gemcitabine (GEM) in pancreatic cancer treatment. Using immunohistochemical evaluation, we investigated that decreased expression of human equilibrative nucleoside transporter-1 (hENT1, which is the major GEM transporter across cell membranes) and increased expression of ribonucleotide reductase subunit 2 (RRM2, which decreases the cytotoxicity of GEM) was associated with low GEM chemosensitivity. To solve these problems, we employed a nanomedicine-based formulation of cationic liposomes for co-delivery of GEM along with siRNA targeting RRM2. Due to the specific endocytic uptake mechanism of nanocarriers and genesilencing effect of RRM2 siRNA, this nanomedicine formulation significantly increased GEM chemosensitivity in tumor models of genetically engineered Panc1 cells with low hENT1 or high RRM2 expression. Moreover, in a series of patient-derived cancer cells, we demonstrated that the therapeutic benefits of the nanomedicine formulations were associated with the expression levels of hENT1 and RRM2. In summary, we found that the essential factors of GEM chemosensitivity were the expression levels of hENT1 and RRM2, and synthesized nanoformulations can overcome these problems. This unique design of nanomedicine not only provides a universal platform to enhance chemosensitivity but also contributes to the precision design and personalized treatment in nanomedicine.
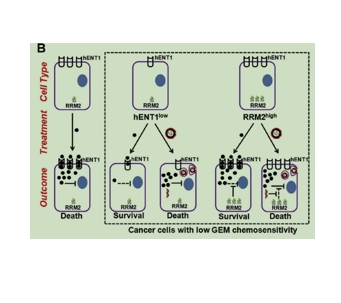
吉西他滨(GEM)对pancreatic cancer有效果。通过免疫组化评估,研究人平衡核苷转运体-1(hENT1,这是跨细胞膜的主要GEM转运体)的表达降低和核糖核酸还原酶亚基2(RRM2,降低GEM的细胞有害性)的表达增加与低GEM化学敏感性降低有关。为了解决这些问题,采用了一种基于DOTAP纳米药物的阳离子脂质体配方,与靶向RRM2的siRNA共同递送GEM。
具体制备纳米配方的合成:
用DOTAP、二油酰磷脂酰乙醇胺、胆固醇、distearoylphosphatidylethanolamine-methyl-polyethyleneglycol偶联物-2000溶解于二氯甲烷,干燥成薄膜,然后用双蒸馏水进行水化过程,形成多层囊泡(MLV)。对于NP-GEM的合成,在水化溶液中加 GEM。然后使用脂质法迷你挤出机将得到的MLV挤出的聚碳酸酯膜,经过循环,形成大的单层囊泡(LUVs)。对于NP-GEM的制备,luv通过超滤离心管离心,以去除未封装的GEM。NP-GEM在双蒸馏水中重悬。对GEM进行脱乳和HPLC分析确定包封效果,其中a为脱乳后NP-GEM中的GEM量,b为初始添加的GEM量。当NP和NP-GEM吸收siRNA时,纳米颗粒溶液与siRNA:1结合,室温下孵育。

图为:使用二油酰基三甲基丙烷铵(DOTAP)为基础的阳离子脂质体纳米颗粒(NP)将GEM封装到载体的亲水核(NP-GEM)中。
纳米配方的表征:
为了进行形态学测量,将纳米颗粒沉积在碳包覆的铜网格上,然后用醋酸铀酰阴性染色,并用透射电子显微镜检查。对于尺寸分布和zeta电位测量,动态光散射(DLS)估计使用激光器和激光器。将DNA与NP-GEM在不同质量比下混合,在室温下DOTAP将混合物用琼脂糖凝胶进行凝胶电泳分析,用SYBR染色,并在紫外光下观察。
图为:采用脂质膜分散法组装基于DOTAP的阳离子脂质体,在制备过程中将GEM封装成其亲水核心(NP-GEM)。
结论:
NP-GEM具有较强的核酸结合能力。纳米载体的siRRM2吸收siRRM2:DOTAP。与NP-GEM相比,siRRM2吸收的NP-GEM(NP-GEM-siRRM2)在吸收siRNA后表面略有不规则,大小分布差异,由于siRRM2结合,zeta电位改变。

 2024-12-17 作者:lkr 来源:
2024-12-17 作者:lkr 来源:

