文献:
Silica‐coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long‐term cardiac function with magnetic field guidance in rats with myocardial infarction
文献链接:
https://pubmed.ncbi.nlm.nih.gov/30982985/
作者:
B Zhang,H Jiang,J Chen,Q Hu,S Yang,X Liu
相关产品:
二氧化硅四氧化三铁纳米颗粒
原文摘要:Low retention of endothelial progenitor cells (EPCs) in the infarct area has been suggested to be responsible for the poor clinical efficacy of EPC therapy for myocardial infarction (MI). This study aimed to evaluate whether magnetized EPCs guided through an external magnetic field could augment the aggregation of EPCs in an ischemia area, thereby enhancing therapeutic efficacy. EPCs from male rats were isolated and labeled with silica‐coated magnetic iron oxide nanoparticles to form magnetized EPCs.
Then, the proliferation,migration,vascularization, and cytophenotypic markers of magnetized EPCs were analyzed. Afterward, the magnetized EPCs (1 × 106 ) were transplanted into a female rat model of MI via the tail vein at 7 days after MI with or without the guidance of an external magnet above the infarct area. Cardiac function, myocardial fibrosis, and the apoptosis of cardiomyocytes were observed at 4 weeks after treatment. In addition, EPC retention and the angiogenesis of ischemic myocardium were evaluated. Labeling with magnetic nanoparticles exhibited minimal influence to the biological functions of EPCs. The transplantation of magnetized EPCs guided by an external magnet significantly improved the cardiac function, decreased infarction size, and reduced myocardial apoptosis in MI rats. Moreover, enhanced aggregations of magnetized EPCs in the infarcted border zone were observed in rats with external magnet‐guided transplantation, accompanied by the significantly increased density of microvessels and upregulated the expression of proangiogenic factors, when compared with non‐external‐magnet‐guided rats. The magnetic field‐guided transplantation of magnetized EPCs was associated with the enhanced aggregation of EPCs in the infarcted border zone, thereby improving the therapeutic efficacy of MI.
二氧化硅包被的磁性纳米颗粒在纳米材料标记中的应用较广,在实际情况中EPCs的保留率较低建议对EPC效果不佳负责MI,所以通过磁化EPCs在外部磁场的引导下,可以增加EPCs在缺血区域,从而提高效率。实验原理为雄性大鼠的EPCs用二氧化硅涂层磁性氧化铁纳米粒子隔离和标记以形成磁化EPCs。然后分析磁性EPCs的增殖、迁移、Blood vessels化和细胞表型标志物。
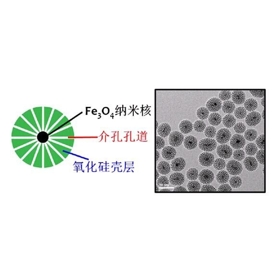
图为:介孔二氧化硅包裹超顺四氧化三铁纳米颗粒
硅涂层磁性氧化铁纳米颗粒标记具体制备:
使用纳米颗粒直径为60 nm,中心为40nm的四氧化三铁核,外表面周围为二氧化硅层。用PBS洗涤贴壁EPCs,并与纳米颗粒溶解到无血清的内皮基础培养基-2(EBM-2)中,不同浓度孵育。为了确定不同剂量下纳米颗粒的标记效率及其对细胞活力的影响,分别采用了普鲁士蓝染色和流式细胞术检测。对于普鲁士蓝染色,在共孵育结束时,用玻璃罩上标记和未标记的EPCs用甲醛固定。然后,用由亚铁氰化钾和盐酸溶液组成的混合溶液以等比例染色。然后,将细胞核用细胞核快速红进行反染。通过Annexin V-FITC和碘化丙啶双染色检测与不同浓度的纳米颗粒孵育的EPCs的活力。轻轻刮取标记的EPCs,收集到离心管中,用电镜固定液在室温下固定。然后用分级酒精脱水,透射电镜观察超薄切片。
细胞表型分析。为了确定与二氧化硅涂层磁性氧化铁纳米颗粒的掺入是否影响了EPCs的表型,在孵育后检测标记和未标记EPCs的细胞表面标记物。两组EPCs被消化和重悬。随后,将这些细胞与CD133抗体(兔多克隆CD133抗体;PE、NB120-16518PE)、CD34(大鼠单克隆CD34抗体、FITC;NB600-1071F)和VEGFR(兔多克隆VEGFR抗体;NB100-2382AF647)一起孵育。然后,通过荧光激活细胞分选(FACS;FACSCalibince,NJ)分析这些生物标记物的百分比。
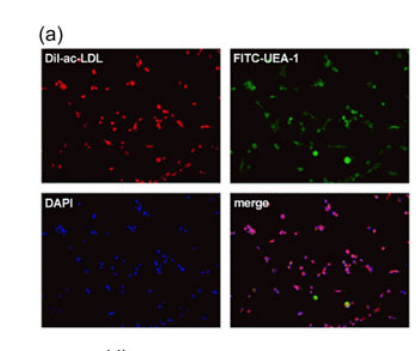
图为:(EPCs)鉴定的代表性图像
结论:二氧化硅磁性氧化铁纳米颗粒对EPCs进行标记,其有效整合,外磁引导下磁化EPCs的潜在效果。此外,纳米颗粒对EPCs的增殖、迁移、管形成、表型和分泌能力没有明显的不利影响。

 2024-12-17 作者:lkr 来源:
2024-12-17 作者:lkr 来源:

