文献:
Polyproline-Polyornithine Diblock Copolymers with Inherent Mitochondria Tropism
文献链接:
https://pubmed.ncbi.nlm.nih.gov/39797465/
作者:
Camilla Pegoraro, Ekaterina Karpova, Yusuf Qutbuddin, Esther Masiá Sanchis, Pavels Dimitrijevs , Cristián Huck-Iriart, Svetozar Gavrilović, Pavel Arsenyan, Petra Schwille, Carles Felip-León, Aroa Duro-Castano, Inmaculada Conejos-Sanchez,María J Vicent
相关产品:
原文摘要:
Mitochondria play critical roles in regulating cell fate, with dysfunction correlating with the development of multiple diseases, emphasizing the need for engineered nanomedicines that cross biological barriers. Said nanomedicines often target fluctuating mitochondrial properties and/or present inefficient/insufficient cytosolic delivery (resulting in poor overall activity), while many require complex synthetic procedures involving targeting residues (hindering clinical translation). The synthesis/characterization of polypeptide-based cell penetrating diblock copolymers of poly-L-ornithine (PLO) and polyproline (PLP) (PLOn-PLPm, n:m ratio 1:3) are described as mitochondria-targeting nanocarriers. Synthesis involves a simple two-step methodology based on N-carboxyanhydride ring-opening polymerization, with the scale-up optimization using a “design of experiments” approach. The molecular mechanisms behind targetability and therapeutic activity are investigated through physical/biological processes for diblock copolymers themselves or as targeting moieties in a poly-L-glutamic (PGA)-based conjugate. Diblock copolymers prompt rapid cell entry via energy-independent mechanisms and recognize mitochondria through the mitochondria-specific phospholipid cardiolipin (CL). Stimuli-driven conditions and mitochondria polarization dynamics, which decrease efficacy depending on disease type/stage, do not compromise diblock copolymer uptake/targetability. Diblock copolymers exhibit inherent concentration-dependent anti-tumorigenic activity at the mitochondrial level. The diblock copolymer conjugate possesses improved safety, significant cell penetration, and mitochondrial accumulation via cardiolipin recognition. These findings may support the development of efficient and safe mitochondrial-targeting nanomedicines
纳米药物通常针对波动的线粒体特性和/或呈现低效/不足的胞质传递(导致整体活性较差),而许多药物需要复杂的合成程序,基于本文合成结果发现聚脯氨酸-聚鸟氨酸 PLO-PLP可按照一定比例成为新的线粒体靶向纳米载体。具体制备如下:
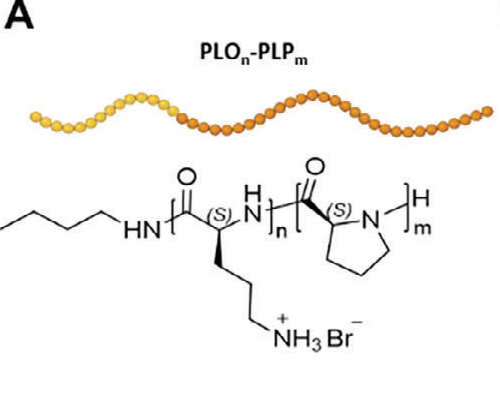
通过改变多阳离子块(PLL、PHis或PArg而不是PLO)来引入PLOn-PLPm平台的变化双块长度(n:m比值)来解读每个组分对线粒体靶向的贡献。双嵌段共聚物6b、h和i(n:m比为1:3)和纳米偶联物16促进了非神经细胞快速进入和有效的线粒体靶向(例如,作为mt-CPOs),从而克服了细胞内吞进入后内吞/溶酶体腔室的问题,这些问题依赖于低效的内/溶酶体破坏机制进行胞质传递。PLO是一种有效的多阳离子阻滞,比一种众所周知的细胞穿透肽PLL促进更快速的细胞内化和的线粒体积累。二嵌段共聚物6b和7-相同的n:m比和长度,但不同的聚阳离子块(PLO和PLL)具有相当的尺寸、电荷和II型螺旋结构。PLO-PLP二块在溶液中呈现单模分布,而PLL-PLP二块由于其有利于随机螺旋-螺旋构象转变,降低了螺旋度(影响溶剂化环境和水壳相互作用),导致不同的水化半径。
实验结果表面聚l-鸟氨酸的多肽基细胞穿透二嵌段共聚物(PLO)和PLP)聚脯氨酸(PLOn-PLPm,n:m比率1:3)的合成/表征被描述为线粒体靶向纳米载体。合成包括一种简单的基于n-羧酸酐开环聚合的两步方法,并采用“实验设计”的方法进行放大优化。通过二嵌段共聚物本身的物理/生物过程,或作为聚l-谷氨酸(PGA)的结合物,研究了靶向性的分子机制。

 2025-03-25 作者:wff 来源:
2025-03-25 作者:wff 来源:

