文献:Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy.
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=5d97f707ec0444ff0e0b667ed3881707&site=xueshu_se
作者:Lei Hua, Zhen Wang, Liang Zhao, Honglin Mao, Guanghui Wang, Kairuo Zhang, Xuejiao Liu,
Dongmei Wu, Yuanlin Zheng, Jun Lu, Rutong Yu, and Hongmei Liu
相关产品:DSPE-PEG2000-PDP 磷脂-聚乙二醇2000-PDP
原文摘要:Treatment of malignant glioma is a challenge facing cancer therapy. In addition to surgery, and chemotherapy, radiotherapy (RT) is one of the most effective modalities of glioma treatment. However, there are two crucial challenges for RT facing malignant glioma therapy: first, gliomas are known to be resistant to radiation due to their intratumoral hypoxia; second, radiosensitizers may exhibit a lack of target specificity, which may cause a lower concentration of radiosensitizers in tumors and toxic side effects in normal tissues. Thus, novel angiopep-2-lipid-poly-(metronidazoles)n (ALP-(MIs)n) hypoxic radiosensitizer-polyprodrug nanoparticles (NPs) were designed to enhance the radiosensitizing effect on
gliomas.
Methods: In this study, different degrees and biodegradabilites of hypoxic radiosensitizer MIs-based polyprodrug (P-(MIs)n) were synthesized as a hydrophobic core. P-(MIs)n were mixed with DSPE-PEG2000, angiopep-2-DSPE-PEG2000 and lecithin to self-assemble ALP-(MIs)n through a single-step nanoprecipitation method. The ALP-(MIs)n encapsulate doxorubicin (DOX)
(ALP-(MIs)n/DOX) and provoke the release of DOX under hypoxic conditions for glioma chemo- and radiotherapy. In vivo glioma targeting was tested in an orthotopic glioma using live animal fluorescence/bioluminescence imaging. The effect on sensitization to RT of ALP-(MIs)n and the combination of chemotherapy and RT of ALP-(MIs)n/DOX for glioma treatment were also
investigated both in vitro and in vivo.
Results: ALP-(MIs)n/DOX effectively accumulated in gliomas and could reach the hypoxic glioma site after systemic in vivo administration. These ALP-(MIs)n showed a significant radiosensitizing effect on gliomas and realized combination chemotherapy and RT for glioma treatment both in vitro and in vivo.
Conclusions: In summary, we constructed a lipid-poly-(hypoxic radiosensitized polyprodrug)
nanoparticles for enhancing the RT sensitivity of gliomas and achieving the combination of radiation and chemotherapy for gliomas.
DSPE-PEG2000-PDP是将DSPE-PEG2000与PDP(吡啶基二硫代丙酰胺)相结合。DSPE-PEG2000中,DSPE具有疏水特性,能与脂类物质相容,而PEG2000链赋予材料良好的亲水性和在水性环境中的稳定性,减少其在体内被免疫系统识别和清除的几率。PDP基团则为材料带来了特殊的反应活性。 这种材料在化合物递送和生物共轭领域有重要价值。它可用于构建先进的化合物输送体系,将化合物分子有效装载于其形成的纳米结构内。其PDP基团可与含巯基的分子发生特异性反应,便于进一步修饰和功能化,比如连接特定的靶向分子、蛋白等,实现准确的靶向给药。基于此该文献设计制备了脂质多聚(ALP-(MIs)n)纳米颗粒,过程如下:
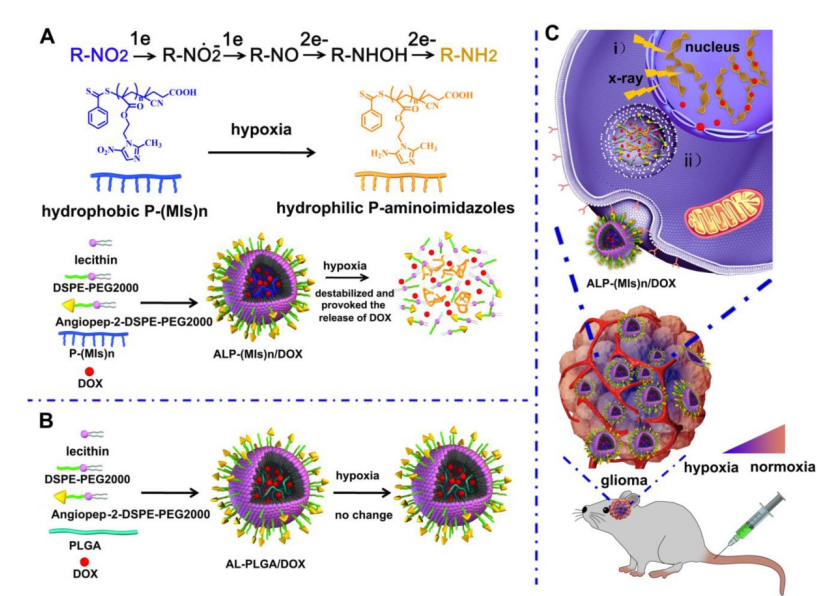
图:缺氧反应和缺氧逆转录酶增敏ALP-(MIs)n给药系统示意图
MI-MA的制备
将甲硝唑(MI)、甲基丙烯酸(MA)和4-二甲胺吡啶(DMAP)溶解于二氯甲烷(DCM)中,置于三颈圆底烧瓶中,附于慢加装置。然后,在氩气流动下搅拌混合物,然后滴注加入二环己基碳二亚胺(DCC,溶解在DCM中)。将混合物搅拌过夜。该混合物被冷却到室温并进行过滤。滤饼用DCM洗涤。将滤液混合,用水洗涤。然后,用无水硫酸钠干燥有机层,并进行减压浓缩。产品经硅胶柱层析得到MI-MA。
聚(MI)n的制备
聚合是在氩气保护下进行的烘烤的施伦克管中进行的。分别加入MI-MA、V-501、CPADB和DMSO。该溶液通过三个标准的冷冻泵解冻循环进行脱氧。然后,将反应管置于预设温度为油浴中。将反应管置入冷却水浴中。加入甲醇后的聚合物以橙油的形式出现,真空干燥。
angiopep-2-DSPE-PEG2000的制备
在DMSO中加入相同摩尔的DSPE-PEG2000-PDP和agiopep-2。将反应混合物在室温下轻轻搅拌。测定了释放的2-吡啶硫酮,对所得产物进行了表征表征。将分辨率冻干得到最终产物angiopep-2-DSPE-PEG2000.根据总脂质(卵磷脂:DSPE-PEG2000:DSPE-PEG2000-2)/P-(MIs)n一定重量比进行调整。
ALP(MIs)n和ALP(MIs)n/DOX的制备
DSPE-PEG2000、卵磷脂、angiopep-2-DSPE-PEG2000、P-(MIs)n在DMSO中完全溶解。将混合物加在一起,剧烈涡旋,在剧烈搅拌条件下滴入水溶液中,然后在室温下轻轻搅拌,形成ALP-(MIs)n. DOX。将盐酸溶于DMSO中,加入三乙胺去除盐酸盐。DSPE-PEG2000、卵磷脂、angiopep-2-DSPE-PEG2000、P-(MIs)n和DOX在DMSO中完全溶解。加入ALP-(MIs)n/DOX,制备ALP-(MIs)n/DOX。将混合物加入在一起,剧烈涡旋,在剧烈搅拌条件下滴入水溶液中,然后在室温下轻轻搅拌形成ALP-(MIs)n/DOX。然后,用PBS(pH 7.4)透析纯化ALP-(MIs)n和ALP-(MIs)n/DOX,以去除游离的DOX和DMSO。
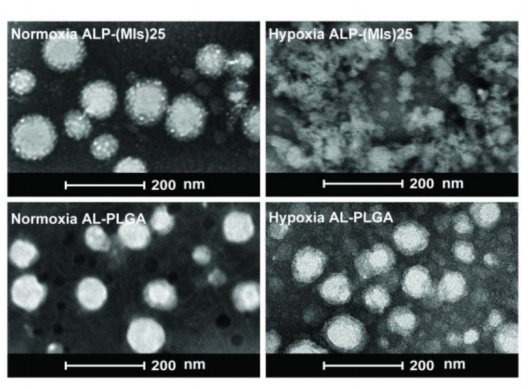
图:常氧和低氧条件下ALP-(MIs)25和AL-PLGA的透射电镜图像
结论:实验得到了缺氧反应多前药NP,具有放疗缺氧致敏作用。DSPE-PEG2000-PDP参与制备的ALP-(MIs)n多前药NPs的RT缺氧致敏作用可提供疏水Chemotherapy ,同时实现效果,并在缺氧条件下诱导化合物的释放。体外和体内实验结果表明,这些ALP-(MIs)n多前药NPs可以有效靶向,并阻止tumor cell生长。

 2025-02-17 作者:ZJ 来源:
2025-02-17 作者:ZJ 来源:

