文献:PEGylated Self-Assembled Nano-Bacitracin A: Probing the Antibacterial Mechanism and Real-Time Tracing of Target Delivery in Vivo
文献链接:https://pubmed.ncbi.nlm.nih.gov/29516722/
作者:Wei Hong,Yining Zhao,Yuru Guo,Chengcheng Huang,Peng Qiu,Jia Zhu, Chun Chu,Hong Shiand Mingchun Liu
相关产品:PEG-PLGA-PEG 聚乙二醇-聚乳酸羟基乳酸共聚物-聚乙二醇
原文摘要:Although nano-self-assemblies of hydrophobicmodified bacitracin A with poly(D,L-lactic-co-glycolic acid)(PLGA) (nano-BAPLGA) have demonstrated promising antibacterial activities, the application of nano-BAPLGA was severely compromised by low water solubility. In this study, a series of PEGylated PLGA copolymers were selected to conjugate with
the N-terminus of bacitracin A to construct PEGylated selfassembled nano-BAs and to further develop nano-selfassemblies of bacitracin A with strong antibacterial potency and high solubility. Compared with nano-BAPLGA, all PEGylated nano-BAs, except nano-BA5k, exhibited strong
antibacterial efficiency against both Gram-positive and Gramnegative bacteria by inducing loss of cytoplasmic membrane potential, membrane permeabilization, and leakage of calcein from
artificial cell membranes. Studies elucidating the underlying mechanism of PEGylated nano-BAs against Gram-negative bacteria indicated that the strong hydrophobic and van der Waals interactions between PLGA and lipopolysaccharide (LPS) could bind, neutralize, and disassociate LPS, facilitating cellular uptake of the nanoparticles, which could destabilize the membrane, resulting in cell death. Moreover, PEGylated nano-BAs (nano-BA12k) with a longer PLGA block were expected to occupy a higher local density of BA mass on the surface and result in stronger hydrophobic and van der Waals interactions with LPS, which were responsible for the enhanced antibacterial activity against Gram-positive and emerging antibacterial activity against Gramnegative bacteria, respectively. In vivo imaging verified that PEGylated nano-BAs exhibited higher inflammatory tissue distribution and longer circulation time than nano-BAPLGA. Therefore, although PEGylation did not affect antibacterial activity, it is necessary for target delivery and resistance to clearance of the observed PEGylated nano-BAs. In vivo, nano-BA12k also showed
the highest therapeutic index against infection burden in a mouse thigh infection model among the tested formulations, which showed good correlation with the in vitro results. In conclusion, nano-BA12k showed high efficacy in the treatment of invasive infections. This new approach of constructing nanoantibiotics by modification of commercially available antibiotics with PEGylated copolymers is safe, cost-effective, and environmentally friendly.
PEG-PLGA-PEG是一种由聚乙二醇(PEG)、聚乳酸-羟基乙酸共聚物(PLGA)通过特定方式连接而成具有特殊结构的高分子材料。这种材料具有良好的生物相容性和可降解性。PEG部分赋予了材料亲水性和抗蛋白吸附性能,减少了与生物体的非特异性相互作用。PLGA则提供了一定的机械强度和降解特性,使其能够在体内逐渐降解为小分子产物。PEG-PLGA-PEG可通过自组装形成纳米胶束,有效地包裹和负载各种化合物分子。在体内运输过程中,保护化合物免受酶解等破坏,并且能够实现化合物的缓释控制释放,提高化合物效果和降低副作用。研究选择一系列聚乙二醇化PLGA共聚物与Bacitracin A的n端偶联,构建聚乙二醇化自组装纳米bas,进一步开发具有高溶解度的Bacitracin A纳米自组装物。过程如下:
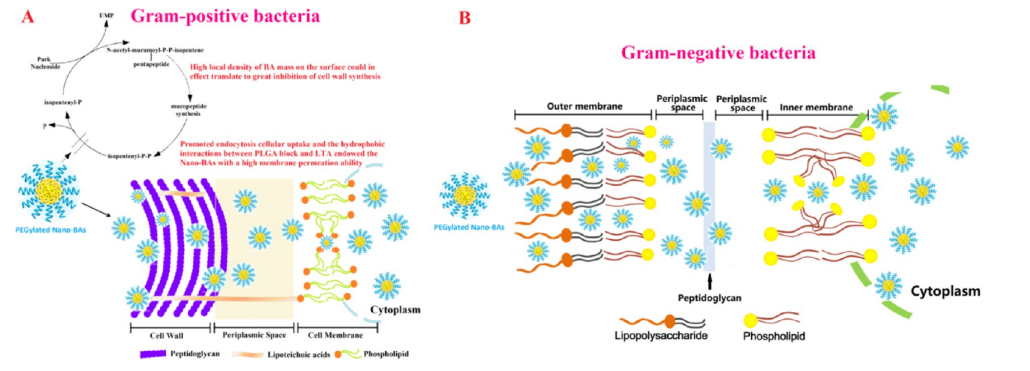
图:聚乙二醇化纳米bas对革兰氏阳性菌(A)和革兰氏阴性菌(B).的作用模式示意图
薄膜水化法制备PEG-PLGA-PEG链接Bacitracin A
将 PEG-PLGA-PEG 聚合物溶解在适量的有机溶剂(如二氯甲烷)中,称取一定量的 PEG-PLGA-PEG 聚合物,放入圆底烧瓶中,加入适量的二氯甲烷,使其充分溶解。将Bacitracin A 溶解在相同的有机溶剂中配制成合适浓度的溶液。然后将Bacitracin A 溶液缓慢加入到上述聚合物溶液中,在搅拌或涡旋条件下使其混合均匀。将混合溶液转移到旋转蒸发仪的茄形瓶中,在一定的温度和转速下,缓慢旋转蒸发去除有机溶剂。随着有机溶剂的蒸发,聚合物和Bacitracin A 会在茄形瓶内壁形成一层均匀的薄膜。向形成薄膜的茄形瓶中加入适量的超纯水,水的量要根据聚合物和Bacitracin A 的总量以及所需的纳米粒浓度来确定。将茄形瓶置于一定温度的水浴中,在温和搅拌或超声条件下进行水化以促进薄膜充分水化并使聚合物和Bacitracin A 形成纳米粒结构。水化完成后,将得到的纳米粒溶液转移至透析袋中透析,以去除未包封的Bacitracin A 和残留的有机溶剂等杂质。透析结束后,将纳米粒溶液取出,通过离心等方法进一步分离和收集纳米粒。收集沉淀的纳米粒,并用适量的超纯水或其他合适的缓冲液重新悬浮,即可得到 PEG-PLGA-PEG 链接Bacitracin A 的纳米粒制剂。
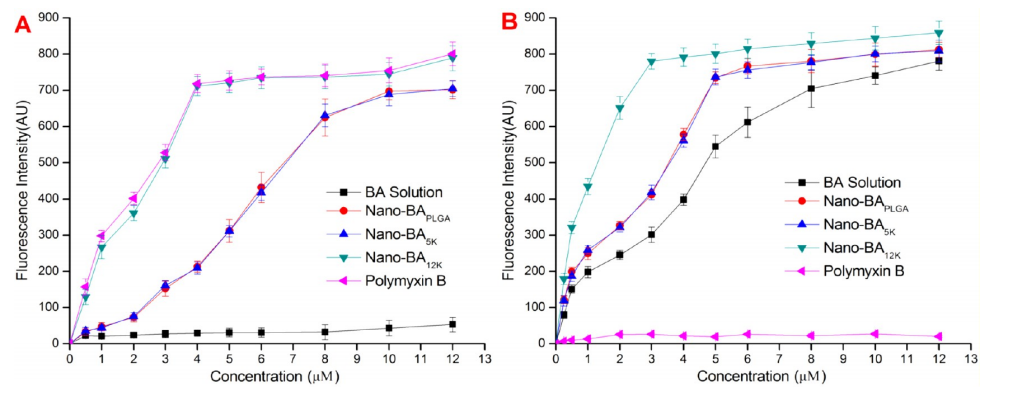
图:纳米bas的外膜通透性。
结论:聚乙二醇化纳米ba,并对其体外和体内效果进行了评价。所有聚乙二醇化纳米ba具有效果,其活性的增强可能是由于纳米颗粒的形成,从而增加了BA质量的局部密度。聚乙二醇化纳米ba的活性可能是由于PLGA与LPS的相互作用。聚乙二醇化的纳米bas通过与脂多糖结合,可导致细胞质膜去极化和细胞质膜完整性的破坏。

 2025-02-17 作者:ZJ 来源:
2025-02-17 作者:ZJ 来源:

