文献:A Novel CD133- and EpCAM-Targeted Liposome With Redox-Responsive Properties Capable of Synergistically Eliminating Liver Cancer Stem Cells
文献链接:https://pubmed.ncbi.nlm.nih.gov/32850663/
作者:Zihua Wang, Mengqi Sun , Wang Li, Linyang Fan, Ying Zhou, Zhiyuan Hu
相关产品:DSPE-SS-PEG- Mal 磷脂-双硫键-聚乙二醇-马来亚酰胺
原文摘要:Cancer stem cells (CSCs) are a small subset of cells that sit atop the hierarchical ladder
in many cancer types. Liver CSCs have been associated with high chemoresistance and recurrence rates in hepatocellular carcinoma (HCC). However, as of yet, no satisfactorily effective liver CSC-targeted treatment is available, which drove us to design and investigate the efficacy of a liposome-based delivery system. Here, we introduce a redox-triggered dual-targeted liposome, CEP-LP@S/D, capable of co-delivering doxorubicin (Dox) and salinomycin (Sal) for the synergistic treatment of liver cancer. This system is based on the association of CD133- and EpCAM-targeted peptides to form Y-shaped CEP ligands that were anchored to the surface of the liposome and
allowed the selective targeting of CD133+ EpCAM+ liver CSCs. After arriving to the CSCs, the CEP-LP@S/D liposome undergoes endocytosis to the cytoplasm, where a high concentration of glutathione (GSH) breaks its disulfide bonds, thereby degrading the liposome. This then induces a rapid release of Dox and Sal to synergistically inhibit tumor growth. Notably, this effect occurs through Dox-induced apoptosis and concurrent lysosomal iron sequestration by Sal. Interestingly, both in vitro and in vivo studies indicated that our GSH-responsive co-delivery system not only effectively enhanced CSC targeting but also eliminated the non-CSC faction, thereby exhibiting high antitumor efficacy. We believe that the smart liposome nanocarrier-based co-delivery system is a promising strategy to combat liver cancer, which may also lay the groundwork for more enhanced approaches to target other cancer types as well.
DSPE-SS-PEG 在正常生理环境下较为稳定,但当处于细胞内含有高浓度还原剂(如谷胱甘肽)的环境中时,双硫键会发生断裂。这种特性使得 DSPE-SS-PEG 在化合物递送系统中具有重要应用价值,比如可以实现化合物的靶向释放。它既能够利用磷脂的特性将化合物包裹在脂质体中,又能借助 PEG 的性质提高化合物的稳定性和生物相容性,还能通过双硫键的断裂响应细胞内环境,准确地释放化合物,提高效果引用的这篇文献基于脂质体的传递系统,介绍了一种氧化还原触发的双靶向脂质体CEP-LP@S/D,具体如下:
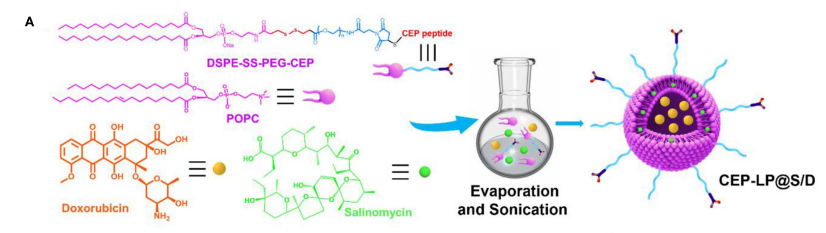
图:CEP-LP@S/D脂质体的结构和形成示意图
分别筛选了EP1(YEVHTYYLD)和CY(ἧple)作为EpCAM和CD133的特异性肽。这两个肽配体通过GG连接体连接,形成y型肽(CEP),由Fmoc固相合成技术合成。然后,通过添加巯基和马来酰亚胺,将CEP共价偶联到DSPE-SS-PEG2000- Mal上。制备脂质体。简单地说,将POPC/DSPE-SS-PEG2000-CEP混合并溶解在氯仿/甲醇中。然后,将一定量的Sal和阿霉素在室温下溶解在甲醇中,并与脂质溶液混合。混合溶剂旋转蒸发干燥,形成脂膜。然后,用磷酸盐缓冲盐水水合干燥的膜并超声。最后,制备的负载药脂质体CEP-LP@S/D通过过滤膜过滤器,以去除任何沉淀物。
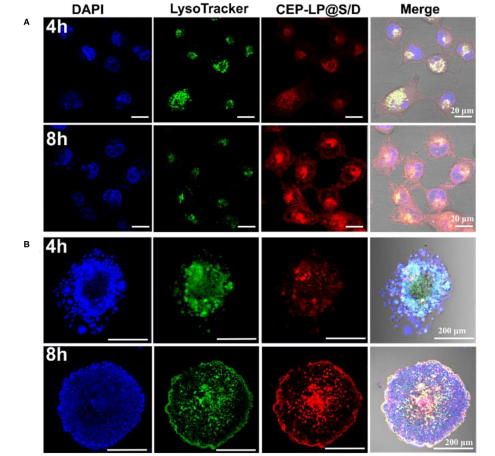
图:通过共聚焦激光扫描显微镜(CLSM)成像获得的CEP-LP@S/D吸收
结论:DSPE-SS-PEG参与制备的CEP-LP@S/D,能够共同递送阿霉素(Dox)和盐碱霉素(Sal)协同应用,该系统基于CD133-和epCAM靶向肽的结合,形成y型CEP配体,这些配体锚定在脂质体表面,并允许选择性靶向CD133+ EpCAM+ CSCs。到达CSCs后,CEP-LP@S/D脂质体进入细胞质内吞,高浓度的谷胱甘肽(GSH)破坏其二硫键,从而降解脂质体。从而诱导阿霉素和Sal的快速释放。GSH应答共传递系统不仅有效增强了CSC的靶向性,而且消除了非CSC派系。

 2025-02-14 作者:ZJ 来源:
2025-02-14 作者:ZJ 来源:

