文献:Visualized podocyte-targeting and focused ultrasound responsive glucocorticoid nano-delivery system against immune-associated nephropathy without glucocorticoid side effect
文献链接:https://pubmed.ncbi.nlm.nih.gov/33456566/
作者:Kui Fan, Li Zeng, Jing Guo, Shuqin Xie, Yuan Yu, Jianwei Chen, Jin Cao, Qinyanqiu Xiang, Siliang Zhang, Yuanli Luo, Qingyue Deng, Qin Zhou, Yan Zhao, Lan Hao, Zhigang Wangand Ling Zhong
原文摘要:Glucocorticoids are widely used in the treatment of nephritis, however, its dose-dependent side effects, such as the increased risk of infection and metabolic disturbances, hamper its clinical use. This study reports a visualized podocyte-targeting and focused ultrasound responsive glucocorticoid nano-delivery system (named as Dex/PFP@LIPs-BMS-α), which specific delivers dexamethasone (Dex) to podocyte targets and reduces systemic side effects.
Methods: The glucocorticoid nano-delivery system was synthesized by a lipid thin film and a simple facile acoustic-emulsification method. This glucocorticoid nano-delivery system used BMS-470539 (BMS-α), a synthetic compound, as a “navigator” to specifically identify and target the melanocortin-1 receptor (MC-1R) on podocytes. The loaded perfluoropentane (PFP) realizes the directed "explosion effect" throughultrasound-targeted microbubble destruction (UTMD) technology under the coordination of low intensity focused ultrasound (LIFU) to completely release Dex.
Results: Both in vitro and in vivo experiments have demonstrated that Dex/PFP@LIPs-BMs-α accurately gathered to podocyte targets and improved podocyte morphology. Moreover, in vivo, proteinuria and serum creatinine levels were significantly reduced in the group treated with Dex/PFP@LIPs-BMS-α, and no severe side effects were detected. Furthermore,
Dex/PFP@LIPs-BMS-α, with capabilities of ultrasound, photoacoustic and fluorescence imaging, provided individualized visual guidance and the monitoring of treatment.
Conclusion: This study provides a promising strategy of Dex/PFP@LIPs-BMS-α as effective and safe against immune-associated nephropathy.
DSPE-PEG-COOH在化学构成上,它融合了DSPE、PEG和羧基(COOH)。DSPE是一种磷脂类物质,其特殊的分子结构使得该化合物具有出色的两亲性,有助于在水性环境中形成稳定的结构,如脂质体。PEG链具有高度的亲水性和柔韧性,可有效改善分子的溶解性和分散性,同时减少在体内应用时与蛋白的非特异性结合,延长循环时间。COOH基团则为分子带来了丰富的化学反应活性,它可以与含有氨基等活性基团的化合物、生物分子进行共价连接,从而实现化合物的靶向修饰、蛋白质的标记或功能化等多种用途。引用的文献以此制备糖皮质激素纳米递送系统(Dex/PFP@LIPs-BMS-α),该系统特异性地将地塞米松(Dex)递送到足细胞靶点。过程如下:
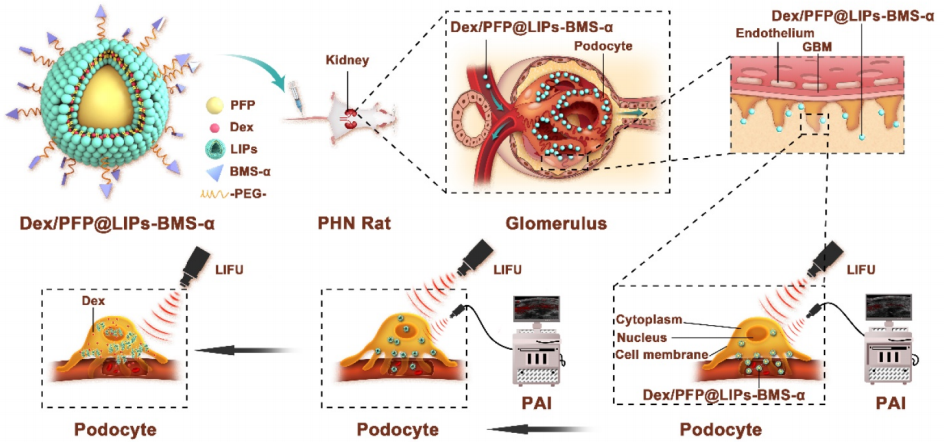
图:Dex/PFP@LIPs-BMS-α的结构
制备Dex/PFP@LIPs-BMS-α
用碳二亚胺法将BMS-α与DSPE-PEG-COOH结合。将 DSPE-微酚溶解在 N-N二甲基甲酰胺(DMF)和无水1-羟基苯甲三唑(HOBt)的混合物中。其中加入N-N-二异丙基碳二亚胺(DIC),在室温下搅拌。然后将BMS-α溶解在 DMF中,室温搅拌。最后,通过BMS-α和DSPE-PEGCOOH脱水合成得到目标产物(DSPE-PEG-BMS-α),并通过高效液相色谱(HPLC)进行鉴定和纯化。采用脂质薄膜和简单简便的声乳化方法合成了糖皮质激素纳米递送系统。首先,将混合脂类和右美托咪定的摩尔比溶解在氯甲烷的混合物中(三氯甲烷)和甲醇(甲醇)。将混合溶液转移到一个圆底烧瓶中,水浴中连续旋转蒸发,形成融合的脂膜。然后,加入PBS,充分补水融合的脂膜,并将混合物转移到冰浴中的 PE管中。然后,加入的PFP。使用超声波探头在冰浴中超声。获得了Dex/PFP@LIPsBMS-α的糖皮质激素纳米传递系统。
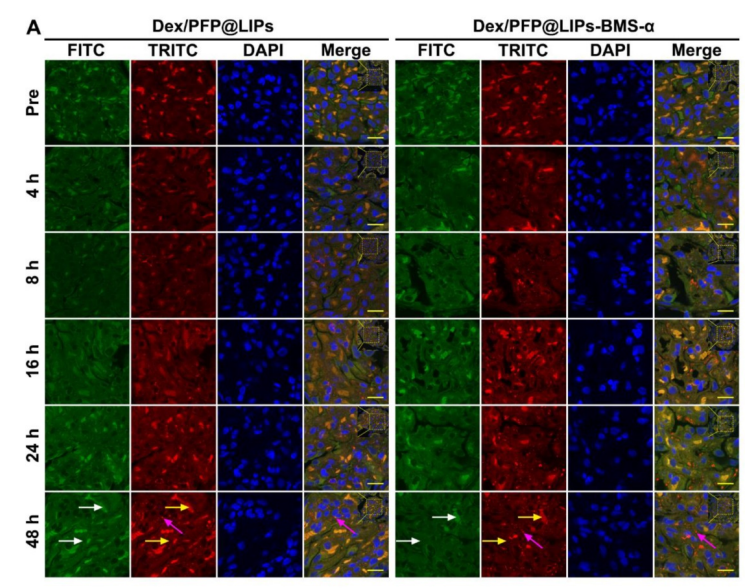
图:Ps-BMS-α通过不同时间间隔(Pre、4、8、16、24、48 h)的细胞定位。
结论:DSPE-PEG-COOH参与制备的细胞靶向聚焦超声响应糖皮质激素纳米传递系统Dex/PFP@LIPs-BMS-α联合LIFU,证明了其作为免疫相关应用的有效性和安全性。这种糖皮质激素纳米递送系统对身体具有较高的亲和力,从而避免了传统的全身糖皮质激素应用的副作用。此外,在Dex/PFP@LIPs-BMS-α中添加LIFU可以提供可视化监测,包括超声和光声监测,以及驱动可控的化合物释放。

 2025-02-14 作者:ZJ 来源:
2025-02-14 作者:ZJ 来源:

