文献:"Sweet tooth"-oriented SN38 prodrug delivery nanoplatform for targeted gastric cancer therapy
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=1k3y0rf0dj420gp0g1020vf080059151&site=xueshu_se
作者:Ning Ding, Shengjun Xu,Sheng Zheng, Qianwei Ye,Li Xu,Sunbin Ling,Shanshan Xie,Wenwen Chen,Zizhen Zhang,Meng Xue, Zhenghua Lin,Xiao Xuand Liangjing Wang
相关产品:DSPE-PEG2000-Glu 磷脂-聚乙二醇2000-葡萄糖
原文摘要:Most cancer cells employ overexpression of glucose transports (GLUTs) to satisfy glucose demand (‘‘Sweet Tooth’’) for increased aerobic glycolysis rates. GLUT1, one of the most widely expressed GLUTs in numerous cancers, was identified as a prognosis-related biomarker of gastric cancer via tissue array analysis. Herein, a ‘‘Sweet Tooth’’-oriented SN38 prodrug delivery nanoplatform (Glu-SNP) was developed for targeted gastric cancer therapy. For this purpose, a SN38-derived prodrug (PLA-SN38) was synthesized by tethering
7-ethyl-10-hydroxycamptothecin (SN38) to biocompatible polylactic acid (PLA) with the
appropriate degree of polymerization (n = 44). The PLA-SN38 conjugate was further assembled with glycosylated amphiphilic lipid to obtain glucosamine-decorated nanoparticles (Glu-SNP).
Glu-SNP exhibited potent antitumor efficiency both in vitro and in vivo through enhanced cancer
cell-specific targeting associated with the overexpression of GLUT1, which provides a promising
approach for gastric cancer therapy.
DSPE-PEG2000-Glu由三部分组成。DSPE(二硬脂酰基磷脂酰乙醇胺)是亲脂性成分,能与脂质膜相互作用,赋予材料在脂质环境中的稳定性和亲和性。PEG2000(聚乙二醇,分子量2000)是亲水性链段,不仅增加了整个分子的水溶性,还能减少蛋白质等生物大分子的非特异性吸附,延长其在体内循环的时间。葡萄糖部分则赋予了分子对特定生物过程或细胞的识别能力,例如可能与细胞表面的葡萄糖转运蛋白等相互作用。可用于化合物递送系统,通过葡萄糖靶向作用将化合物运输到特定细胞,也可用于生物成像等方面。该文献通过将7-乙基-10-羟基喜树碱(SN38)与适当的聚合度聚乳酸(n = 44)结合,合成了SN38衍生的(PLA-SN38)。进一步将PLA-SN38偶联物与糖基化的两亲性脂质进行组装,得到了葡萄糖胺修饰的纳米颗粒(Glu-SNP)。
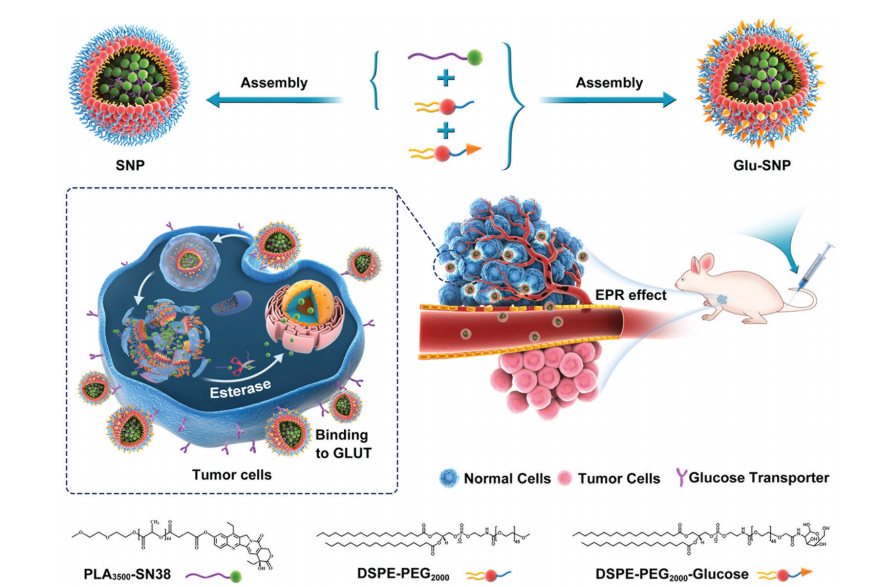
图:sn38负载纳米颗粒(SNP和Glu-SNP)的化学结构和组装过程示意图。
SN38前药纳米颗粒的制备
PLA-SN38(SN38等量)与DSPE-PEG2000摩尔比预混。而在制备葡萄糖胺修饰的SN38前药负载纳米颗粒(Glu-SNP)的过程中,PLA-SN38(SN38等效)与两种基质(DSPE-PEG2000和DSPE-PEG2000-Glu)的摩尔比固定。将预定量的DSPE-PEG2000-Glu和/或DSPEPEG2000和PLA-SN38溶解在丙酮中,然后滴加入去离子水中,同时搅拌。在室温下搅拌后,通过减压旋转蒸发去除剩余的丙酮。进一步浓缩纳米颗粒溶液,并用去离子水洗涤以进行进一步实验。采用动态光散射法测量了SNP和Glu-SNP的水动力直径和zeta电位。
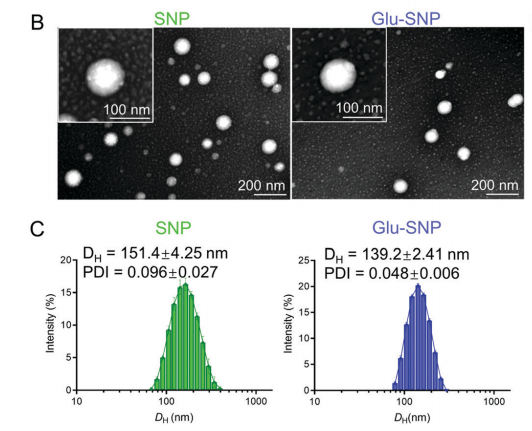
图:具有代表性的透射电子显微镜(TEM)图像、SNP和Glu-SNP的水动力直径(DH)分布和多分散性指数(PDI)
结论:DSPE-PEG2000-Glu参与制备的SNP和Glu-SNP均具有适当的粒径、长期稳定性和持久的化合物释放谱。与未修饰的纳米颗粒相比,纳米颗粒的葡萄糖修饰通过体外glut1特异性的细胞内积累增强了细胞有害性。由于EPR效应的适当利用和葡萄糖装饰的主动靶向,Glu-SNP表现出良好积累,避免了可能的脱靶效应。

 2025-02-14 作者:ZJ 来源:
2025-02-14 作者:ZJ 来源:

