文献:A Cleverly Designed Novel Lipid Nanosystem: Targeted Retention, Controlled Visual Drug Release, and Cascade Amplification Therapy for Mammary Carcinoma in vitro
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=136m0t50hb170xy0ef4u0v10xb020720&site=xueshu_se
作者:Xiang-Zhi Zhao ,Wei Zhang, Yang Cao,Shuai-Shuai Huang,Yi-Zhen Li ,Dan Guo, Xing-Yue,Wang, Hai-Tao Ran
相关产品:DSPE&C-RGD 磷脂&C-RGD
原文摘要:Objective: To construct an ideal theranostic nanoplatform (LIP3); to clarify its physico
chemical properties; to confirm its characteristics of dual-modality imaging, active-targeting, and cascade amplification therapy for mammary carcinoma; and to perform a preliminary exploration of the cytotoxicity mechanism.
Design: A self-prepared liposome nanosystem, LIP3, can actively target 4T1 cells because the surface is linked with C-RGD. Haematoporphyrin monomethyl ether (HMME), an excellent sonosensitizer entrapped in the lipid bilayer, can function in photoacoustic imaging. Low-intensity focused ultrasound (LIFU) of ultrasound-targeted microbubble destruction (UTMD) promotes localized drug delivery into tumours because PFH, a phase-change substance, is loaded in the LIP3 core, achieving visualization of targeted drug release, and sonodynamic therapy (SDT) can kill tumour cells. SDT provides a favourable environment for AQ4N, resulting in amplification of LIP3 treatment. Therefore, LIP3 shows targeted aggregation and targeted release, integrating dual-mode imaging and precise treatment.
Results: The self-prepared lipid nanosystem, LIP3, meets the above expectations and has ideal physicochemical properties, with a regular sphere with uniform distribution. Contrastenhanced ultrasound (CEUS), photoacoustic imaging, and bimodal imaging were effective in vitro. In 4T1 cell experiments, the cell capacity was as high as 42.9%, and the cytotoxicity to 4T1 was more than 5 times that of LIP1 (containing AQ4N only) and more than 2 times that of LIP2 (containing only HMME), achieving comparable results as cascade therapy for mammary cancer.
Conclusion: LIP3, a theranostic nanoplatform, was successfully constructed and conformed to the physicochemical characterization of ideal nanoparticles, with active-targeting, dualmodality imaging, visualized drug release, and precise treatment under the action of LIFU. SDT provides a favourable environment for AQ4N, resulting in amplification of LIP3 treatment. Therefore, LIP3 shows targeted aggregation and targeted release, integrating dualmode imaging, and precise cascade treatment. This unique theranostic NPS with multiple capabilities is expected to be a favourable anti-cancer method in the future.
DSPE&C-RGD是一种复合物。DSPE(1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺)是一种亲脂性成分,有助于其与细胞膜等脂质结构相互作用。C-RGD则是含有精氨酸-甘氨酸-天冬氨酸(RGD)序列的环肽。RGD序列是一种被认可的与细胞表面整合素特异性结合的靶向基团。DSPE&C-RGD这种组合将脂质的物理化学性质与RGD的靶向功能相结合,它可以引导整个复合物与表达相应整合素的细胞进行特异性结合,用于细胞靶。基于此,引用的这篇文献设计自制备的脂质纳米体系LIP3。过程如下:
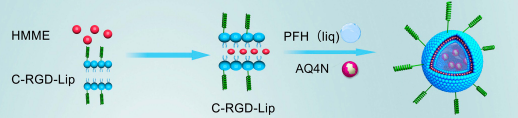
图:LIP3的合成过程
LIP0、LIP1、LIP2、LIP3的制备
采用传统的膜色散-超声振荡法,合成过程如图所示。简单地说,混合DSPE&C-RGD和脂溶性化合物根据特定的比例进行称重(DPPC:DPPG:DSPE&C-RGD/DSPE:CHOL:HMME=质量比),溶解在有机溶剂中,下一步用旋转蒸发器去除,直到形成均匀的薄膜。然后,用磷酸盐缓冲盐水(PBS)溶解。然后,加入PFH和AQ4N。最后,用超声器进行超声振荡,获得DSPE&C-RGD,并进行低温离心。所有过程均在荧光荧光环境中进行。LIP1是指C-RGD肽纳米颗粒平台装载AQ4N和PFH;LIP2装载PFH和HMME;LIP3装载PFH、AQ4N和HMME;LIP0是另一个LIP3,即没有连接到LIP3的目标(C-RGD)。
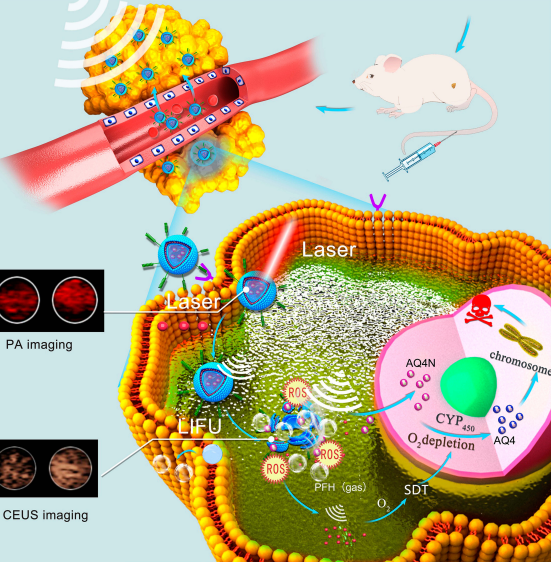
图:LIP3双峰成像与Treatment 的机制原理图
结论:实验得到DSPE&C-RGD与RGD的靶向功能相结合得到脂质体纳米系统LIP3,LIP3表现出靶向聚集和靶向释放,整合了双模式成像。具有规则的球面和均匀分布。SDT为AQ4N提供了良好的环境,导致LIP3处理的扩增。对比增强超声(CEUS)、光声成像和双峰成像在体外都是有效的。在4T1细胞实验中,细胞容量高达42.9%,对4T1的细胞有害性是LIP1(仅含AQ4N)的5倍以上,是LIP2(仅含HMME)的2倍以上,达到作用效果。

 2025-02-13 作者:ZJ 来源:
2025-02-13 作者:ZJ 来源:

