文献:Nanocarriers of Fe3O4 as a Novel Method for Delivery of the Antineoplastic Agent Doxorubicin Into HeLa Cells in vitro
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=116v06h0hx700jw0mg5402s01b612798&site=xueshu_se
作者:Kun-kun Xia, Yong Lyu2, Wei-tang Yuan , Gui-xian Wang , Harrison Stratton , Shui-jun Zhang and Jie Wu
相关产品: DSPE-SS-mPEG 磷脂-双硫键-甲氧基聚乙二醇
原文摘要:Here we report the synthesis and in vitro characterization of a redox-sensitive, magnetically inducible nanoparticle carrier system based on the doxorubicin (DOX) drug delivery model. Each quantal nanocarrier unit consists of a magnetite Fe3O4 nanoparticle core that is further encapsulated in self-assembled micelles of the redox-responsive polyethylene glycol derivative, DSPE-SS-mPEG. The nanocarrier system was prepared using a combination of ultrasonication and dialysis to produce the microenvironment sensitive delivery system. The final synthesized and DOX-loaded magnetic nanocarriers had an average size of ∼150 nm when assembled with a 6.9% DOX payload. The release rate of DOX from these redox-responsive magnetic nanocarriers was shown to be accelerated in vitro when in the presence of glutathione (GSH). Furthermore, we demonstrated that more redox-responsive magnetic nanocarriers could be taken up by HeLa cells when a local magnetic field was applied. Once internalized within a cell,
the micelles of the outer nanocarrier complex were broken down in the presence of higher concentrations of GSH, which accelerated the release of DOX. This produces a particle with dual operating characteristics that can be controlled via a specific cellular environment coupled with an exogenously applied signal in the form of a magnetic field triggering release.
DSPE-SS-mPEG是由DSPE(1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺)、可还原的二硫键(SS)和甲氧基聚乙二醇(mPEG)组成的高分子材料。DSPE赋予了该材料良好的脂质特性,使其具有亲脂性,能够与生物膜等脂质环境良好地相互作用。在特定的还原环境下,比如细胞内的某些具有还原能力的区域,二硫键能够发生断裂,从而实现材料结构和性能的改变,实现化合物的靶向释放等功能。mPEG链段则提供了材料的亲水性和良好的生物相容性。它可以减少蛋白质的吸附,降低材料在体内的免疫原性,使整个分子在生理环境中更稳定。基于DSPE-SS-mPEG的性质,研究每个量子纳米载体单元由一个磁铁矿四氧化三铁纳米颗粒核组成,该核进一步封装在氧化还原响应性聚乙二醇衍生物DSPE-SS-mPEG的自组装胶束中。采用超声和透析相结合的方法制备了纳米载体系统,制备了微环境敏感传递系统。过程如下:
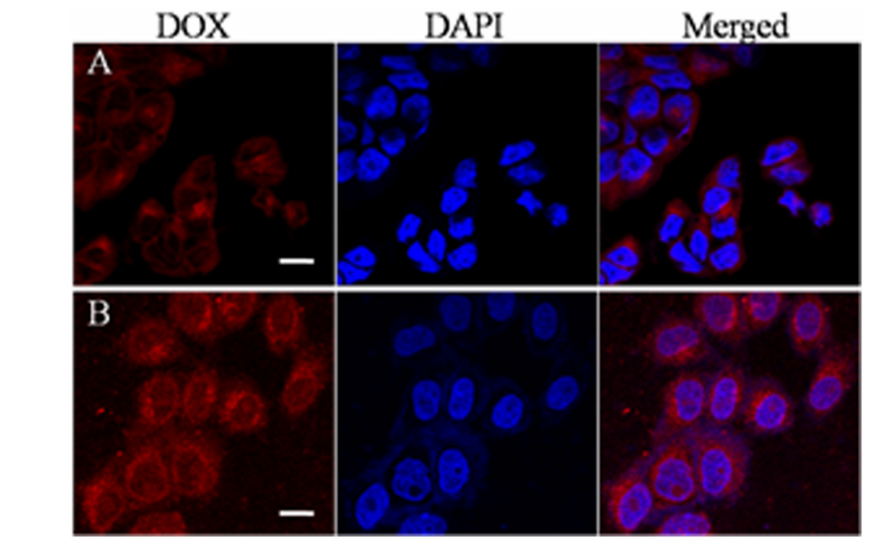
图:在没有(A)或有(B)磁场条件下,HeLa细胞经dox负载的磁性纳米载体处理2小时后的CLSM图像。
纳米载体的制备
采用超声波-透析法制备了负载dox的氧化还原响应性磁性纳米载体。简单地说,将DOX·HCl在DMSO中加入两倍摩尔数的TEA,搅拌,获得DOX碱。在溶液中加入DSPE-SS-mPEG,在室温下再搅拌。同时,将四氧化三铁纳米颗粒溶解在(四氢呋喃)THF中。将上述两种溶液混合,经超声处理加入超纯水中。然后将混合溶液转移到透析管中,在室温下用超纯水透析数小时。
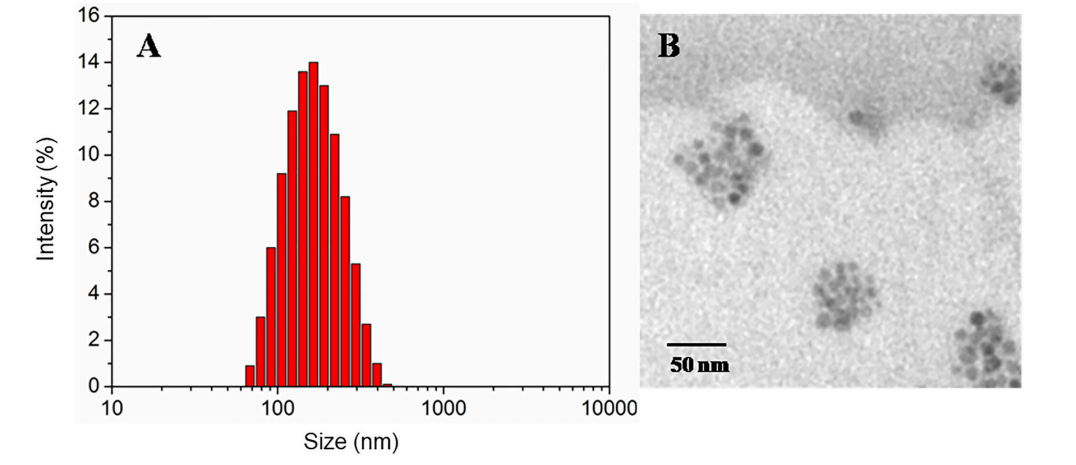
图:DLS (A)、TEM(B)对氧化还原响应磁性纳米载体的尺寸分布
结论:最终DSPE-SS-mPEG参与合成的DOX负载磁性纳米载流子在6.9% DOX负载组装时,平均尺寸为∼150nm。在体外,当谷胱甘肽(GSH)存在时,这些氧化还原响应性磁性纳米载体中DOX的释放速率加快。此外,当施加局部磁场时,HeLa细胞可以吸收更多的氧化还原响应磁性纳米载体。一旦在细胞内内化,外部纳米载体复合物的胶束在更高浓度的GSH存在下被分解,这加速了DOX的释放。这就产生了一种具有双重工作特性的粒子,它可以通过特定的细胞环境和磁场触发释放形式的外源性应用信号来控制。

 2025-02-13 作者:ZJ 来源:
2025-02-13 作者:ZJ 来源:

