文献:Targeted combination therapy for glioblastoma by co-delivery of doxorubicin, YAP-siRNA and gold nanorods
文献链接:https://xueshu.baidu.com/usercenter/paper/show?paperid=12760pc0hx1c0030gx560e0002069257&site=xueshu_se
作者:Lihuang Lia,Yanxiu Liub,Jun Yangc,Qiang Zhanga , Mindan Lub , Xiumin Wangb,Liangcheng Lib,and Lei Ren
相关产品:DSPE-PEG2000-MAL 磷脂-聚乙二醇2000-马来亚酰胺
原文摘要:The combination of brain targeting drug delivery systems and multi-modalintervention pose a promising therapeutic approach for glioblastoma therapy. In this study, we developed an angiopep-2 peptide modified cationic liposomes loaded with doxorubicin, YAP-siRNA and gold nanorods (D/R@Ang2-Lip+Au) simultaneously, which has been characterized by high encapsulating efficiency for doxorubicin (95.4%) and effective binding of siRNA at N/P ratio of 20:1. The fluorescence imaging and flow cytometry analysis revealed high cellular uptake of
D/R@Ang2-Lip+Au. Real-time quantitative polymerase chain reaction and western blot analysis indicated that D/R@Ang2-Lip+Au could effectively inhibit the expression of YAP protein. In vitro and in vivo studies showed that D/R@Ang2-Lip+Au had the ability to target glioma cells, and achieved better anti-proliferative effects compared with non-targeted D/R@Lip+Au. Moreover, in
vivo experiment demonstrated that D/R@Ang2-Lip+Au was able to cross the blood-brain barrier, and combination therapy could significantly inhibit tumor growth. Therefore, the multifunctional D/R@Ang2-Lip+Au might provide a novel approach for effectively delivery of DOX, AuNRs and siRNA into the glioblastoma cells simultaneously and exerting synergistic therapeutic effects.
DSPE-PEG2000-MAL是一种功能化的磷脂-聚乙二醇衍生物。DSPE是一种常见的磷脂成分,赋予分子两亲性,使其能够在水性环境中自组装形成胶束或与细胞膜相互作用。中间的 PEG2000 链段具有高度的亲水性和良好的柔韧性,可有效改善分子的水溶性,并减少在生物体内的非特异性吸附。末端的马来酰亚胺(MAL)基团则具有很高的化学反应活性,能与含有巯基(- SH)的生物分子(如蛋白质、多肽)发生特异性的 Michael 加成反应。这种化合物在化合物递送系统中可用于制备脂质体,将化合物包裹在内部实现靶向运输;在生物标记领域,可通过与目标生物分子结合,实现对生物分子的标记和追踪。引用的这篇文献研究了一种同时装载阿霉素、YAP-siRNA和金纳米棒的多肽修饰阳离子脂质体(D/R@Ang2-Lip+Au),过程如下:
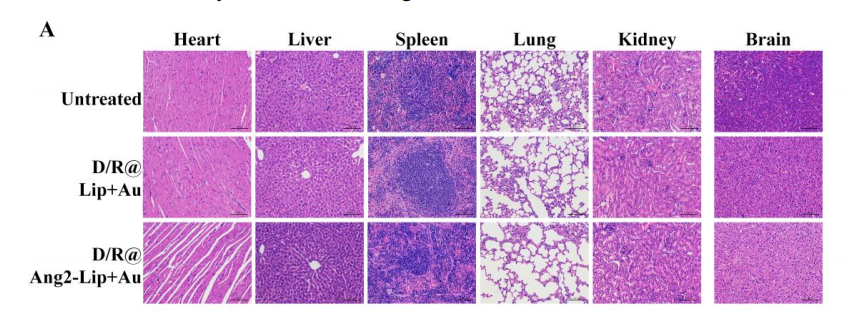
图:实验染色图形
用薄膜水化法制备阳离子脂质体
将脂质,包括DOTAP、DOPE、HSPC和Chol以一定摩尔比用氯仿溶解,在真空下旋转蒸发形成薄膜,然后用(NH4)2SO4水合,之后,用超声波产生均匀的单层脂质体。最后,用插入后技术制备了Blood vessels蛋白-2修饰的阳离子脂质体。即将DSPE-PEG2000-MAL加入脂质体中,孵育。
制备aunr和DOX共载脂质体(DOX@Lip+Au)
通过脂质体或脂质体-Blood vessels蛋白孵育,Aunr与脂质体(Lip+Au)复合物。一定重量比的Lip+Au和DOX孵育,通过透析膜分离游离DOX。对于AuNR、DOX和siRNA共载脂质体(D/R@Lip+Au),DOX@Lip+Au与siRNA以N/P混合。然后,室温孵育,以确保siRNA有效加载。
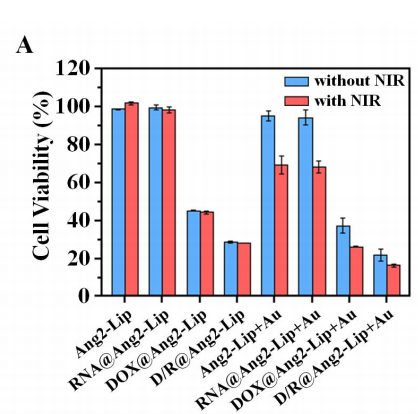
图:用靶向脂质体配方孵育的U87MG细胞的相对细胞活力。
结论:体外细胞摄取实验表明,DSPE-PEG2000-MAL参与制备的D/R@Ang2-Lip+Au可以同时将DOX和siRNA有效地传递到U87MG细胞中。此外,与非靶向D/R@Lip+Au相比,靶向配体Blood vessels蛋白-2的存在增加了细胞对D/R@Ang2-Lip+Au的摄取。此外,D/R@Ang2-Lip+Au在U87MG细胞中诱导的基因沉默效率高于D/R@Lip+Au。

 2025-02-13 作者:ZJ 来源:
2025-02-13 作者:ZJ 来源:

